Hi everyone!
It’s been a while. This start of the year has been quite busy between starting new collaborations, experiments, analysis and presentations. I can’t complain though, there are many exciting things in queue!
My last post was about cardiac electro-mechanics and some introduction to slice culture. I left you with the idea of taking this let’s call it ex-vivo model to the next step and induce atrial fibrillation (AF).
AF can be clinically defined as random activation of the atria, leading to impaired functionality and ineffective mechanical contraction. However, “random” is used only for simplicity: only because a pattern has not been yet discovered does not mean that it does not exist.
It is noteworthy to keep in mind that AF is characteristic of a multicellular prep. Therefore, a single cell cannot show fibrillation, but only arrhythmic behavior. This may lead and maintain fibrillation, if and only the number of arrhythmogenic cells within the tissue is high enough. This means that it cannot happen, by definition, at a single cell level. The reason why I say “by definition” is that the main mechanism sustaining AF is formation and maintenance of re-entry circuits. Re-entries are spiral waves (like the one showed in the movie below, from Hussaini et al., 2021) which cause the tissue to be re-excited along an abnormal loop-like path.
Without getting in too much detail, in physiological conditions the excitation wave travels along one direction across several cells. When a re-entry is formed, the electrical signal travels along a circuit, which causes “backwards” re-excitation of the tissue. These abnormal waves stimulate the heart to rapid, repetitive and inefficient contraction, giving rise to fibrillation. In other words, to have AF we need re-entries, and to have re-entries we need multiple cells.
Why is it relevant to explain this? My experiments involve different types of preparations, going from single cells, to tissue level. Figure 1 shows a recording of a single cardiac cell displaying weird contraction patterns, which could be misinterpreted for fibrillating activity, when in fact it is just stress-related arrhythmic behavior.
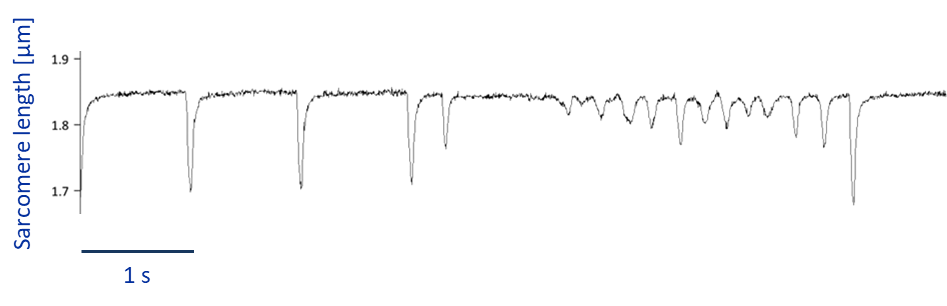
As a comparison, if you have a look at figure 2, you can see what fibrillation is more likely to look like when I culture tissue slices and probably you can also get a hint about why it is commonly defined as ”random”.
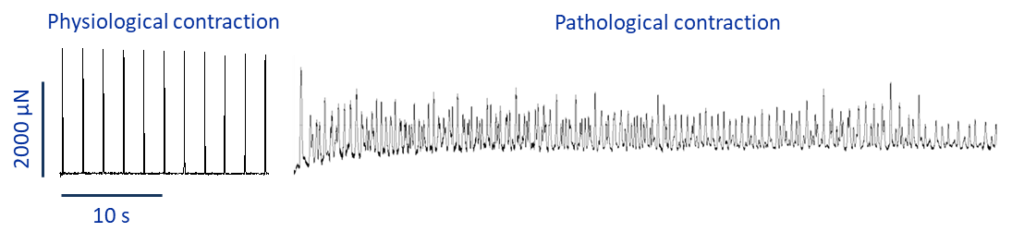
This example, show how powerful an ex-vivo model such as tissue culture is compared to single cells. It allows one to more closely recapitulate what is likely to happen in vivo in a patient-specific manner.
Next time, I’ll tell you how one can try and induce atrial fibrillation!
Teresa
