Myostretching with a view!

In healthy conditions the heart experiences several mechanical changes. Let’s just think about the pressure that the blood exert on the cardiac walls when it enters each chamber, or when it contracts to eject the blood in the systemic flow. When a pathological condition occurs, the mechanical environment undergoes substantial changes and on top of physiological mechanical forces, the tissue experiences acute and chronic pathological stretch which in turn keeps aggravating the condition and so on and so forth in a loop. During atrial fibrillation, islands of fibrotic scar tissue are interspersed within the healthy one. As a consequence, stiffness increases locally and cells experience additional mechanical stimuli, forcing them to re-arrange according to this newly-formed anatomical obstacle, thus inducing further fibrosis development.
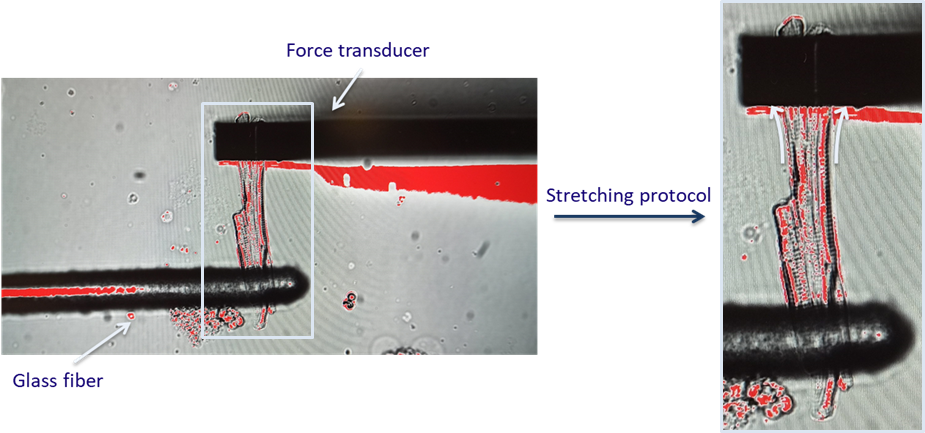
To be able to characterize how cells react to stretch during atrial fibrillation, the MyoStrecher System comes in handy. In simple words, it consists in attaching the cell with two probes, pull them apart and record cell response (Figure 1). Stretch in the atria induces different electrophysiological changes and it is connected with an increase in force production, which makes stretching experiments relevant despite the pathology under investigation. Just to give you an idea of how powerful a tool like this can be, it allows applying a preload and control afterloads, this while imaging a single cell. In general, when we stretch cells, we simulate the effect that the surrounding cells and ECM have on one single cell in the tissue as in their native environment a cell never contracts freely.
You may ask why using single-cells experiments when we are trying to understand how an entire organ works. Well, to be able to characterize the function at the whole-organ level, it is important to have a clear understanding of why intrinsic cellular mechanisms are critical to match cardiac output demands. Single-cell level allows evaluating the interplay between mechanical, electrical and biochemical signalling pathways that underlie all the regulatory mechanisms. It is a bit like with a clock; you know what its function is and that there are mechanisms behind it that work in synchrony and harmony to prevent the lancets from moving too fast or too slow. However, you do not usually ask yourself how this is achieved; you give it for granted and rely on it in order not to be late for work. For cardiac scientists, it is important to understand why and how each component specifically works, so that we can understand the reason that leads to malfunction and impairments without any apparent good reason, such as in the case of fibrillation, when the heart starts running faster than it is supposed to.
But let’s go back to experiments. Using this MyoStretcher is easier said than done, which is why I had a trip to Amsterdam to have a short, thus intensive training. Just a brief recap for those who do not know, the purpose of me using this technique is simulating the variation in stretch that cells experience when there is fibrosis formation and using calcium signalling as readout. If you want to know more about the kind of experiments I am working on, I suggest you have a look at this paper on calcium sparks.
The system consists of a glass fibre connected to a piezoelectric motor, which basically can be programmed to execute a prescribed stretching protocol for length control by changing the electrical waveform (wave-to-wave amplitude and duration). On the other edge of the cell the force transducer is attached via a cantilever. An optical fibre is then used to track the cantilever position which allows deducing the force produced by the cell.
The training was quite productive and if everything goes as planned we should get the whole set-up shipped here by the end of the month, so that I will start stretching cells on a regular basis.
I feel like the quote Alea iacta est fits this post, not because of experiments (it would be a bit extreme), but because of the other exciting experience I had this month. We went hiking and climbing in the Black Forest with some colleagues and supervisors of mine and I did my first via ferrata ever! I have to say that I did not expect to literally hang from a straight wall with no going back. As of right now, it was an extremely exciting experience, but I have to admit that at every turn as the situation was getting less and less stable, spending the day on the couch with a warm blanket sounded more and more appealing (and way safer).

Looking forward to my next climbing adventure though, this time ich werde bereit sein!
Until next post,
Teresa
