Hi there!
As I mentioned in my last post, this post will be dedicated to inducing atrial fibrillation in tissue culture.
If we think about the “macro” triggers which increase the predisposition for fibrillating episodes to happen, this can be due to several risk factors such as comorbidities, emotional triggers or alcohol. Given one of them, what happens at the tissue level?
In general, a functioning heart manages on its own the electrical activity needed for its normal contraction. In other words, this means that given the right conditions, the heart could potentially beat outside of the body. Each heartbeat starts in the right atrium at the sinoatrial node; here, thanks to their automaticity property, pacemaker cells generate and send out the electrical impulse. The video below gives a good idea of this automatic and inherent mechanism (focus on the isolated chunk at the bottom of the dish, you will see how the contraction starts in the central and more transparent part – that’s the sinoatrial node! – and then travels towards the edges).
This impulse will then travel along the heart, provoking orderly contraction of the atria and ventricles (sinus rhythm), finally resulting in blood ejection. However, when fibrillation happens, this is not true anymore. I recently read an interesting article (Denham et al.) which describes the basic mechanisms behind arrhythmias and more specifically AF; I will try to summarize some of the key concepts explained in there.
The arrhythmogenic mechanism underlying AF can be divided in automaticity, triggered activity and re-entries. We saw a nice example of how automaticity basically means that the tissue is able to stimulate itself. Triggered activity is usually referred to as the multiple additional impulses that are triggered by a single initial action potential. In other words, as the electrical impulse travels along one cell, this should be excited and therefore contract once and only once, ensuring the typical physiological contraction of the tissue. However, it may happen that this single action potential encounters a cell having some dysfunctionalities therefore resulting in extra-excitatory events, called afterdepolarizations. Last but not least, as I mentioned in my last post, re-entries are one of the mechanisms underlying AF.
The combination of this 3 mechanisms will create the perfect conditions for maintaining atrial fibrillation. On top of this, AF is associated with a vulnerable atrial substrate which is characterized by extensive electrophysiological, mechanical and structural remodeling of the tissue.
To induce atrial fibrillation in the lab, researchers try to re-create the environmental conditions which are associated with these mechanisms. For example, this remodeling is associated with tissue dilatation, which may be associated with increased tension. This can be simulated by applying stretch to the tissue. Of course, AF is an arrhythmia therefore there must be some electrical stimulation to induce its appearance. What I can do in these terms is apply preload and electrical burst pacing, respectively.
As you can see from the trace in figure 1 the atrial tissue on which I tried this 2 methods simultaneously was quite resilient and recovered nicely without showing any arrhythmic behaviour.
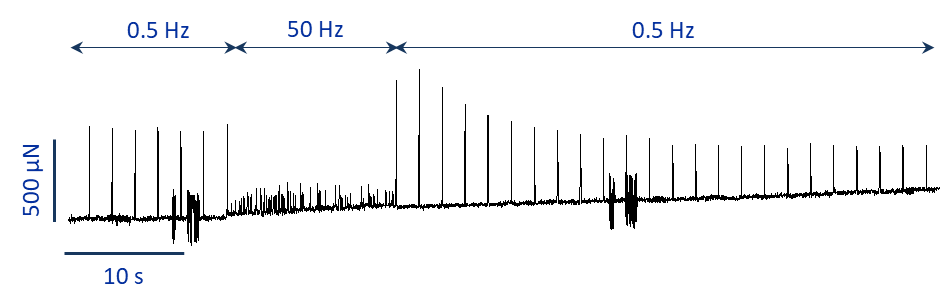
Alternatively, it is possible to induce fibrillation via pharmacological intervention. Drugs directly modify the ionic currents which contribute to the formation of the AP. Therefore, by targeting the right currents within the tissue, obtaining arrhythmic behaviour is more straightforward even with really healthy slices (figure 2).
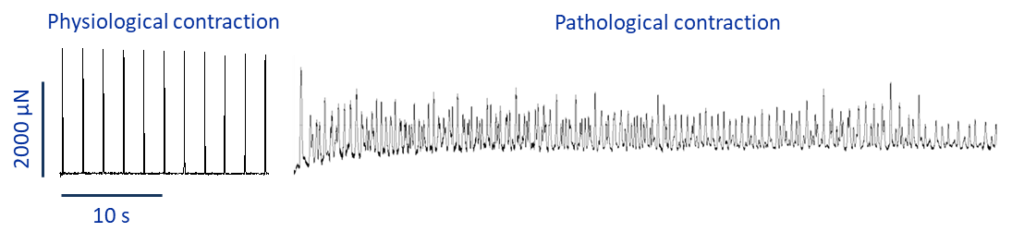
More exciting experiments to come!
Stay tuned,
Teresa
